



East African Stump-tailed Chameleons
By Thomas Hildenhagen
Citation:
Hildenhagen, T. (2007). East African Stump-tailed Chameleons. Chameleons! Online E-Zine, February 2007. (http://www.chameleonnews.com/07FebHildenhagen.html)
Introduction
The African stump-tailed chameleons of the genera Rhampholeon and Rieppeleon comprises 17 species with the vast majority being endemic to tropical East Africa with an epicenter of speciation in Tanzania. The only one of these species recorded from the western and central parts of Africa is Rhampholeon (R.) spectrum. Stump-tailed chameleons, sometimes termed as leaf or pygmy chameleons, are particularly small, often with short tails and usually in shades of grey or brown. They don't have horns but some have rostral processes or a rosette-like appendage (Rhampholeon spinosus) and soft, spiky enlarged scales on flanks and limbs. The ecological information of the East African stump-tailed chameleons is extremely limited. In the last few years the reports of a successful care or breeding of these small reptiles has increased. Many of these animals are still passed on as Rieppeleon kerstenii or Rieppeleon brevicaudatus. This incorrect information reduces the survival of animals drastically and places the future owner with unknown issues.

Currently, the best existing literature sources on these fascinating reptiles is “Stump-tailed Chameleons” by PETR NECAS and WOLFGANG SCHMIDT (2004) and “A Field Guide to the Reptiles of East Africa” by STEPHEN SPAWLS et al (2002).
Distribution
In the following text, the environments and climate of Rhampholeon spinosus (MATSCHIE, 1892), Rhampholeon (Rhampholeon) temporalis (MATSCHIE, 1892), Rhampholeon (Rhinodigitum) moyeri MENEGON, SALVIDIO & TILBURY, 2002 und Rhampholeon (Rhinodigitum) nchisiensis (LOVERIDGE, 1953) from Tanzania and Malawi will be presented. Their distributions reach from the Eastern Arc Mountains in north-eastern Tanzania to the Southern Rift Montane Area in south Tanzania (Southern Highlands) and north-western Malawi. They are found in very limited ranges in the sub-montane and montane forests or forest edges where they are threatened by rapid deforestation. A total of five stump-tailed chameleons species occur in the Usambara Mountains (SPAWLS et al, 2002). Two of these species, R. (R.) spinosus and R. (R.) temporalis, are endemic. The first is common at altitudes up to 2,630 feet (800 m) in the eastern part of the Usambaras and up to 4,270 feet (1,300 m) in the western part of the Usambaras. R. (R.) temporalis only found in the Eastern Usambaras at an altitude up to 2,630 feet (800 m). There are often located close to Amani. The Usambaras are one of the prominent parts of the Eastern Arc Mountains with an altitude at 7,550 feet (2300 m). Being close to the Indian Ocean, they receive higher rainfall at the lower eastern slopes with a yearly average from 1,900 mm at Amani to 2,200 mm at Kwamboro (IVERSEN 1991). The mean humidity in the Eastern Usambaras is 90% in the morning and 75% at midday. Fog and mist has been recorded as much as 130 days annually. In Amani, the mean annual temperature is 69.1°F (20.6°C), the mean daily maximum is 76.8°F (24.9°C) and the mean daily minimum 61.3°F (16.3°C)(IVERSEN 1991). The Western Usambara Mountains are much drier but exact climatic data is less available. The weather station of Mazumbai Estate derived an average rainfall between 1935 and 1985 of 1,400 mm at an altitude of 5,000 feet (1520 m).

Another part of the Eastern Arcs is the Udzungwa Mountains, with the highest peak, Luhombero, with an altitude of 8,460 feet (2,579 m). On the higher slopes at 5,250 feet (1,600 m) where R. (Rd.) moyeri is recorded, the rainfall measures 3,000 mm per year (SHANGALI et al. 1998). The average temperature at this altitude varies from 55.4 to 78.8°F (13 to 26°C) in January with humidity from 65 to 94% (MENEGON et al. 2002). R. (Rd.) moyeri is located in two different areas in the south-western Udzungwas (Mufindi district) - Kihanga Valley at an altitude of 5,840 feet (1,780 m) and Kitolomero Valley 3,850 feet (1,174 m). Both are in the Udzungwa Scarp Forest Reserve (Luhega).

Elevated plateaus and mountains centered on the northwest shores of Lake Malawi are the home of R. (Rd.) nchisiensis. They are a part of the Southern Rift Montane Area with altitudes near 9,840 feet (3,000 m). R. (Rd.) nchisiensis is recorded originally from the lower Nchisi (5,000 feet, 1,520 m) and Misuku Mountains (6,890 feet, 2,100 m) in Malawi. They also recorded from the Poroto, Rungwe, Ukinga Mountains and Kipengere Range in southern Tanzania. It may be found at altitudes of 5,910 feet (1,800 m) and above. One of the differences between this ecoregion and the Eastern Arc Mountains relates to the differing climate regimes. The Southern Highlands receive a mean rainfall of approximately 1,500 mm per year from the surface convection of Lake Malawi. The wet Poroto Mountains have an average annual rainfall of 2,850 mm (CRIBB & LEEDAL 1982). Mean annual temperatures vary from 55.4 to 66.2°F (13 to 19°C)(DOWSETT-LEMAIRE 1989), with an average maximum of 71.6°F (22°C) and an average minimum of 49.6°F (9.8°C)(MCKONE & WALZEM 1994). At the highest areas, frosts are common and temperatures as low as 23°F (-5°C) have been recorded.
Identification
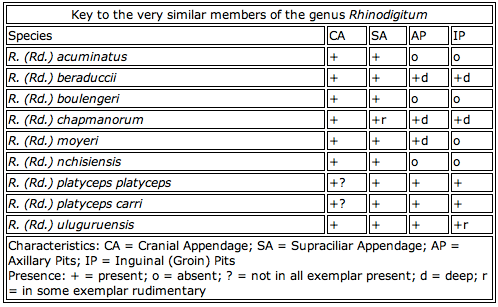
Stump-tailed chameleons are extremely difficult to identify. Some can be easily separated by atypical rostral (cranial) appendage (e.g. R. (R.) spinosus ) or in size (e.g. R. marshalli) but there are few species that look very similar. There may clearly be different in specific appearance, but describing the detailed differences is not easy. Particularly the members of the new subgenus Rhinodigitum have many similarities. Colour pattern is not a consistent character to distinguish the species. Rather there are subtle distinctions in the character of some body parts, e.g. forming of the dorsal crest, direction of the rostral process or presence of axillary dermal depressions (axillary pits). In most species the sex differences find only be determined by the hemipenal bulge and the tail length of the males.

A rough classification between Rieppeleon and Rhampholeon species is possible by the pattern of their side-stripes. Rieppeleon species have stripes across the flanks running horizontal from the head to the tail. Rhampholeon species have two or three diagonal stripes along the flanks running in the anterodorsal to posteroventral direction. The stripes heightening the resemblance of lead veins of dead leaf.

In the genus Rhinodigitum the presence of processes (cranial and supraciliar appendages) and dermal depressions (axillary and inguinal pits) are helpful to identify some species. Cranial or rostral processes are dermal appendages without a bone structure. Mostly they look like a bent digit not longer than 0.1 inch (3mm). The supraciliar appendages consist of one big scale or a cluster of scales that rises up above the eyebrows. Dermal depressions behind the limbs are referred as axillary or inguinal (goin) pits. This small invaginations bear resemblance to the so-called "mite pockets" of other saurian species. They are often not greater than 0.08 inch (2mm) in diameter and at most less than 0.04 inch (1mm) deep. So far there is nothing known about their function.

Table 1

Notes on General Care
Most of the wild-caught stump-tailed chameleons are debilitated and often carry internal parasites (helminthes). Fecals should be performed by a vet as soon as possible to see if treatment is necessary. First enclosure to acclimate should be a simple quarantine-box which is easily to keep clean. After a negative fecal examination, they could move in their regular enclosure. Newly imported animals require a lot of hydration because most of them are dehydrated. Adult wild-caught females are especially delicate as most of them are gravid. They need rest and absolute isolation to acclimate to the new surroundings.

A controversial subject in herpetoculture is whether stump-tailed chameleons should be housed together or individually? Some keepers argue that the interspecific aggression isn’t well developed because pair-wise housed animals rarely display noteworthy reactions. But anytime one animal becomes dominate over another, sooner or later the dominated specimen gets stressed or sick. From my past experiences it is best to keep stump-tailed chameleons individually! Another negative habit is to touch or pet these small chameleons, mainly to activate the buzzing body vibration. This practice will harm them and can cause ongoing stress.

The amount of food consumed will vary with temperatures and climatic conditions. It is best to feed in the morning, because at this time is the greatest active phase. To retain food items in one place, a flat cup can be used. Adults will eat only slightly larger prey items and will not be able to eat anything larger than 20% of their own body size. Uneaten or dead food items in the feeding-cup should be removed daily before they spoil and present a hygiene risk. It is no unusual that crickets bother stump-tailed chameleons on their resting places and give them deep wounds. It is preferred to take excess food items out of the enclosure before the sleep.

Young stump-tailed chameleons should be kept in smaller enclosures to ensure they get the food they require. After hatching the young are transferred directly to small enclosures to come to rest for at least a day, to give them time to absorb the rest of the yolk sac. When adequate moisture is reached, droplets from misting should evaporate in two or three hours. The surface of the soil or leave litter should be allowed to dry out between mistings. Do not overcrowd the stump-tailed chameleons especially the hatchlings.

Rhampholeon (R.) spinosus (MATSCHIE, 1892)
This chameleon had been described as Chamaeleon (Brookesia) spinosus in 1892 by Paul Matschie (1861-1926) and later 1986 Klaver & Böhme named it Bradypodion spinosum. In 2004 Tilbury & Mariaux transfer it to the genus Rhampholeon, and 2006 Mariaux & Tilbury place it now in the subgenus Rhampholeon. This species is currently listed under CITES Appendix II because of his former membership in the genus Bradypodion, indipendant of his new taxanomic status.

Description
The largest male and female I recorded were 3.5 inches (90 mm) and 3.4 inches (85 mm) total length respectively. The tail is thin, partial prehensile and about half the TL. The most distinctive characteristic is the laterally compressed, rosette-shaped dermal appendage, which is soft and movable with enlarged scales. It can reach a length of 0.2 inch (5 mm). In males the rather prominent casque is raised at the back and overlies the dorsal line. Widely spaced, spiny, enlarged scales extend along the back and most of the way along the tail. Rows of soft spine-like scales are present on the flanks, each side of the tail and the limbs. A gular crest is formed by two diverging rows of stumpy scales - a feature also common to the species Chamaeleo (Trioceros) laterispinis, tempeli, incornutus and Calumma capuroni. They also have axillary dermal depressions equivalent to the deep invaginations (axillary and inguinal pits) of some species of the subgenus Rhinodigitum. Males may be distinguished by coarser scales on the appendage and a hemipenal bulge. The coloring of R. (R.) spinosus can be very variable. The color palette ranges from grey, brown and green up to blue, turquoise, orange, red and yellow, with black interstitial skin of the throat. Sometimes dark-colored stripes are present on the flanks.


There are no species in East Africa that look like R. (R.) spinosus. The Malagasy Calumma boettgeri (BOULENGER, 1888) is very similar but lacks enlarged scales on the rostral appendage and the body. The next similar stump-tailed chameleon is R. (Rd.) acuminatus, a new described species from the neighbouring Nguru Mountains, which has a comparable rostral appendage mostly bent down. R. (R.) spinosus are not a shy chameleon; they don't cover behind branches when someone picked up. They keep quiet and trust their perfect mimicry. Rapid body vibration when touched have not been observed like other in other Rhampholeon species.

Habitat
These chameleons inhabit the lower vegetation, bushes, small tress and thicket in the moist, moderate climate montane forests (STEPHAN KALLAS, personal communication). They may be found at night sleeping on outer branches from a few inches to 10 feet (3m) but seldom on the ground. Some other chameleon species living in the same areas include Bradypodion tenue, Ch. (T.) deremensis and R. (R.) temporalis.

Captive Care
The cage for a single animal should be 16 x 16 x 16 inches (40 x 40 x 40 cm). This species is best kept individually, because interspecific aggression is well developed. Adequate ventilation of the cage is crucial. Screen on the top and half of one side frame is standard. Special fluorescent tubes with UV-light are essential for indoor housing. HQL or HQI-lamps are used in larger enclosures with a minimum high of 24 inches (60 cm). Day temperatures should be around 68 to 77°F (20 to 25°C) with night time drops to 61 to 64°F (15 to 18°C). Hydration requirement are high, misting at least twice a day is necessary. Humidity during the day should range from 70 to 95% (after misting), at night 80 to 95%. The cages should be well planted with small foliage plants (e.g. ferns, herbs, tresses) and branches, some with dead leaves. The substrate should be a mix of styrolite-free soil and sand (2:1) covered with some dead leaves, bark or moss. The chameleons readily accept most insects and other arthropods of appropriate size such as small crickets (Acheta domesticus), flies (Fannia canicularis, Lucilia caesar), grey roaches (Nauphoeta cinerea) and waxworms (Galleria mellonella, Achroea grisella). Food items should be dusted with a multi mineral supplement with high-calcium (Miner-all). They lap droplets from the leaves or drink the spray runs down their head.

When the following conditions are met, outdoor setups in moderate climate zones are possible and recommended:
-no direct sunlight! R. (R.) spinosus prefer densely planted enclosures (e.g. Asparagus falcatus)
-full or semi-screened cage in a shady area that only receives a maximum temperature of 77°F (25°C).
-misting during the hottest part of the day to minimize the low moisture outside.

Reproduction
This species is oviparous, like the rest of the genus Rhampholeon. Breeding occurs throughout the year with six or more clutches per year. For mating the male is placed into the female’s terrarium. When the female is receptive she remains quiet and displays light colors. A non receptive female displays dark colors and makes threatening postures to ward off the male. The only observed copulation lasted around 15 minutes. Around four weeks after mating the females lay their eggs in a moist spot, mostly under a bark covered corner. The substrate for egg-laying females needs proper moisture content and should be around 3 inches (75 mm) deep. Sometimes they simply scatter the eggs on the ground of the enclosure. Clutch size ranged from three to four eggs. The eggs are white with a size of 0.43 x 0.24 inches (1.1 x 0.6 cm). Clutches were kept in Vermiculite at room temperature, not in an incubator. The incubation duration lasted about 6 month at temperatures from 64 to 72°F (18 to 22°C) with a light nightly temp drop. For all my Rhampholeon clutches I use small boxes with some holes in the lid for the circulation of air. The appointed water potential at 9g Vermiculite with 12g water is -600 kPa (KÖHLER, 2005). The hatching begins mostly at night or early in the morning and takes a few hours.



Care of Hatchlings
Care for hatchlings of R. (R.) spinosus is very similar to adult care and they can be housed very successfully under the same conditions. Similarly individually housing is essential. The first cage (small spider-tank) should be 8 x 8 x 8 inches (20 x 20 x 20 cm) with tiny twigs or blades of grass. The newborn chameleon’s size is about 1.2 inches (30 mm). Small enclosures will make it easier for young stump-tailed chameleons to find their food. A good item to have in the enclosure is small ferns. These help to retain moisture and also supply a surface for water to collect for the young chameleons to drink. They feed first on flightless fruit flies (Drosophila hydei, D. melanogaster) and pinhead crickets always dusted with calcium supplement. Juvenile seem to be nuts about the slim larvae of the grey roaches. At the age of four months the young chameleons can move in enclosures of the size of the adults. Sexual maturity occurs at approximately 9 months of age.


Rhampholeon (R.) temporalis (MATSCHIE, 1892)
This stump-tailed chameleon had been described as Chamaeleon (Brookesia) temporalis in 1892 by PAUL MATSCHIE and in 1986 KLAVER & BÖHME named it Rhampholeon temporalis. MATTHEE et al (2004) classify it in the subgenus Rhampholeon. This species is currently not listed in any CITES Appendix.

Description
This species has a maximum size of about 3.2 inches (80mm), and an average of 2.0 to 2.6 inches (50 to 60 mm). The short tail is 27 to 36% of total length and not prehensile, even though it may be used for gripping. The casque is flattened and the small, undulated dorsal crest reaches to the end of the tail. Gular and ventral crests are absent, but small spines are present on the throat and the limbs. A small flexible rostral process of enlarged scales rises forward off the snout. The temporal crest ( canthus temporalis ) is only present by two or three enlarged scales. A deep pit is present in each axilla, but none in the goin. Males exhibit a broader tail base. The basic coloration reaches from maroon to grey-brown with two or three dark, diagonal stripes along the flanks. R. (R.) temporalis is very similar to R. (R.) spectrum (BUCHHOLZ, 1874) distinguished by the absence of any prominent canthal processes (supraocular appendage) above each eye.
They have some interesting self-defense strategies. When touched or angered they produce a noticeable body vibration, or fall itself from a branch and play dead.

Habitat
These little chameleons are common in submontane and montane forests and forest edges. They live in low dense vegetation and on the ground in leaf litter. They readily enter well wooded gardens and plantations (NECAS & SCHMIDT, 2004). They are often located close to R. (R.) spinosus (TILBURY & MARIAUX, 2004), but very rarely seen in the same location as Ri. brevicaudatus (EMMETT, 2004).
Captive Care
The intermale aggression of these species is strong, more weakly in interspecific. Individual animals can be kept in well screened enclosures of 16 x 16 x 16 inches (40 x 40 x 40 cm). Most of the day, R. temporalis climbs in the dense vegetation or on the ground. For that reason a full spectrum fluorescent tube should be an adequate lighting with Vitalights or other reptile high-fluorescent lights that produce amounts of UVA and UVB lights even avoided. Temperatures reach day time highs of 77°F (25°C) with night time lows of 61 to 64°F (15 to 18°C). The chameleons require a relative humidity of 70 to 95% that can be maintained by misting the enclosure several times a day. Night time moisture should stay at 80 to 95%. The substrate should be made of a mix of soil and sand (2:1) with a layer of dead leaves. The enclosures should be well planted with dense small plants such as ferns, herbs or little Asparagus. Branches with dead leaves and some overgrown with tresses should be placed for climbing. It is important to provide hiding places like a half cork bark tube covering horizontal a corner, or an abundantly covered corner with vegetation. It will be a retreat area for the animals which will help to reduce stress. Plants and proper substrate are important in maintaining required humidity levels. The main diet consists of insects and other arthropods which include small crickets, grey roaches, flies, firebrats (Thermobia domestica) and waxworms. Food items should be dusted every second feeding with multi mineral supplement (Miner-all) containing Vitamin D3 for proper calcium utilization. They lap droplets from the leaves or drink the water running down their head. Occasionally stump tailed chameleons like R. (R.) temporalis push their head laterally on damp plants or dead leaves and absorb the surface-humidity.

Reproduction
These species is not particular about a breeding season and they will mate throughout the year. They lay their eggs about 40 days after mating, predominantly in the evening hours (SCHMIDT, 2004). The typical clutch size is two or three eggs. R. temporalis clutches have been successfully incubated under the same conditions as R. (R.) spinosus with day temperatures from 64 to 72°F (18 to 22°C) with a weak nightly temperature drop. The egg-laying substrate in the enclosure should be at least 3 inches (75 mm) deep. Gravid females prefer a proper moisture place to laying her eggs, mostly behind a flowerpot or under plant canopy. Eggs are white and dotted with bright spots; the egg size is 0.55 x 0.24 inches (1.4 x 0.6 cm). The incubation duration in Vermiculite lasts from 135 to 150 days. Clutches of WOLFGANG SCHMIDT (2004) incubated successfully from 150 to 172 days at 68 to 73.4°F (20 to 23°C).


Care of Hatchlings
The care of the young R. (R.) temporalis can be housed under the same conditions as the adults. They are best kept individually in enclosures of 8 x 8 x 8 inches (20 x 20 x 20 cm). The neonates measure approximately 1 inch (25 mm). Tiny twigs or blades of grass with a ground of leave litter are the first fittings. A small fern retain moisture and supplies the hatchling with water to drink. Flightless fruit flies and pinhead crickets always coated in calcium supplement should be offered daily, to juveniles every other day. Misting vigorously at least twice a day is ideal. At the age of six months the young chameleons can move in taller enclosures or one the size of the adult’s enclosure. Sexual maturity occurs at approximately 9 to 12 months of age.


Rhampholeon (Rd.) nchisiensis (LOVERIDGE, 1953)
This species had been described as Brookesia nchisiensis in 1953 by ARTHUR LOVERIDGE (1891-1980) and in 1986 KLAVER & BÖHME named it Rhampholeon nchisiensis. In 2004 MATTHEE et al classify it in the new subgenus Rhinodigitum. This species is currently not listed in any CITES Appendix.


Description
Total length of the Malawian species from the Nchisi Mountains reaches a maximum of 3.3 inches (83 mm). Those from the Nkuka Forest in the Rungwe Mountains in Tanzania reach a somewhat smaller total of 2.2 inches (56 mm) (LOVERIDGE, 1953). I have never personally record a R. (Rd.) nchisiensis greater then 2.0 inches (50 mm), and it is my opinion that the majority of specimens imported into Europe are from South Tanzania. Males are distinctly smaller than females with a significant hemipenal bulge. Tail length amounts to about only 20 % of the body length. A small flexible rostral process of a length of 0.08 inch (2 mm) rises forward off the snout. The most unusual feature of the species is the head, which is very flat and recognizable only by the easily raised lateral edges. An undulated dorsal crest is present, but no gular and ventral crests. There is a prominent canthal processes (supraocular appendage) above each eye. Their basic color is grey-brown which with its characteristic two or three dark, diagonal stripes at the flanks. Occasionally a pale green or yellow specimen is seen. Males and females frequently display light blue or turquoise eye turrets, although they are still hard to detect in the leave litter.

R. (Rd.) nchisiensis is closely related to R. (Rd.) moyeri and R. (Rd.) uluguruensis. But R. (Rd.) nchisiensis is easily distinguished by their pitless axilla and goin (table 1). The three species have some interested behavior. When touched or angered they inflate themselves like a highly gravid female. A body vibration is also present in these species. Most of the specimen in the trade have been females, apparently due to the fact that the natives in Tanzania and Malawi who collect the chameleons seem to mostly take the larger females, believing them to more valuable than the smaller males.

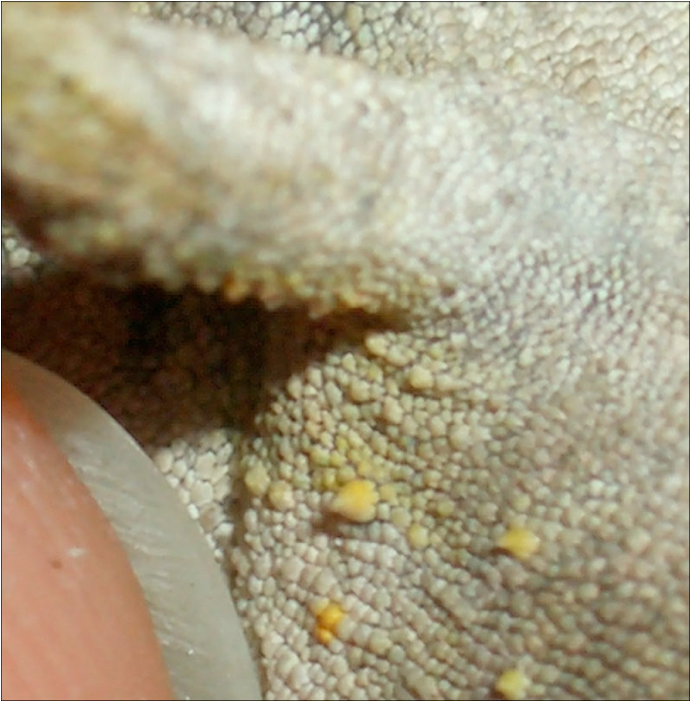
Habitat
This species is located in the remaining stocks of the primary forests and highland grasslands. They live in closed-canopy forests in the valleys and gullies and are also found in the wide open grasslands (HILMAR HUFER, personal communication) on the upper slopes. The chameleons climb into low dense vegetation or on the ground in leaf litter. In some habitats R. (Rd.) nchisiensis is sympatric with a few species of the subgenus Trioceros like Ch. (T.) incornutus in the Ukingas and Ch. (T.) fuelleborni in the Porotos.
Captive Care
These montane species need an enclosure with two or more sides screened. A cage size for an adult of a least 16 x 16 x 16 inches (40 x 40 x 40 cm) will be sufficiently. Interspecific aggression is well developed so it is best to keep this species individually. In order to remain healthy this species needs a high fluctuation between daytime and nighttime temperatures. It should be around 68°F (20°C) during the day with a maximum 73°F (23°C), the nighttime lows under 61°F (16°C). Setups in cooled rooms (air conditioning) or cool cellars are the best. R. (Rd.) nchisiensis are native to an area with high rainfall which they require a high humidity of 75 to 95% during the day and 90 to 100 % at night. It can be maintained by misting three times a day and once more after lights off. Associated with their low light habitat, this species needs dense plants like ferns, herbs or other small foliage plants building a closed canopy. For this reason a simple white fluorescent light to indicate day should suffice. The soil should be covered with a thick layer of dead leaves which the small chameleons can hide under. Substrate should be made of a mix of soil and sand (2:1) and should be at least 3 inches (75 mm) deep. The main diet is insects and other arthropods which include small crickets, grey roaches, flies, firebrats and waxworms. Food items should be dusted every second feeding with multi mineral supplement (Miner-all) contains Vitamin D3 for proper calcium utilization. They lap droplets from the leaves or drink the spray running down their head. Sometimes they absorb the surface-humidity from the damp leaves litter just like R. (R.) temporalis.


Reproduction
It is best to place the male into the females enclosure. When the female is receptive she remains quiet and displays light colors. The male displays dark diagonal stripes and bright blue eye turrets. Copulation lasts from 30 minutes to several hours. Egg laying has been observed all year round with two to four clutches a year after a gestation period from a couple of weeks to 4 month in one case. The females deposit relatively large clutches of 8 to 15 eggs, with a highest number of 19 eggs (ROBERT MASLAK, personal communication). The eggs are white with orange and violet blotches. The size is 0.35 x 0.16 inches (0.9 x 0.4 cm). Some of my females had problems with their egg-laying and became egg-bound. The main reason is the incorrect temperature of the egg-laying substrate. It is essential not let the soil temperatures rise above 66°F (19 °C), and you should try to promote around 59°F (15 °C). Furthermore they prefer a proper moisture place, mostly under plant canopy or bark tube. Sometimes they simply scatter the eggs in the leave litter. An over-soaked substrate is bad too. The eggs from R. (Rd.) nchisiensis will need to be incubated at cool temperatures from 61 to 66°F (16 to 19°C) not higher. Incubation time is surprisingly short and ranges from 65 (ROLF MÜLLER, personal communication) to 104 days.


Care of Hatchlings
Newborn chameleons are extremely small with a total length of 0.6 inch (15 mm). They therefore need a small terrarium at first. They can be raised together until 60 days but do better is housed individually in small enclosures of 4 x 4 x 6 inches (10 x 10 x 15 cm). The remaining care for hatchlings of R. (Rd.) nchisiensis is very similar to the care of the adults and they can be housed very successfully under the same conditions. First fittings should be tiny twigs or blades of grass with a ground of leave litter. Small fern or herbs retain the misting moisture and supplies the hatchling with water to drink. First food items are the small flightless fruit fly (Drosophila melanogaster) and pinhead crickets coated in calcium supplement. The small enclosures should be misted vigorously at least twice a day. At the age of six months the young can move in bigger enclosures (8 x 8 x 8 inches, 20 x 20 x 20 cm), and after 12 months they are the size of the adults. Sexual maturity occurs at approximately 9 to 12 months of age. The difference in the sexes is easily noticeable in the young as females grow faster than the males.

Rhampholeon (Rd.) moyeri MENEGON, SALVIDIO & TILBURY, 2002
This species was recently described in 2002 by MICHELE MENEGON, SEBASTIANO SALVIDIO (both Italy) and COLIN R. TILBURY (South Africa). MATTHEE et al classified it in 2004 in the new subgenus Rhinodigitum. It is currently not listed in any CITES Appendix.


Description
This species closely resembles R. (Rd.) nchisiensis but is actually more closely related to R. (Rd.) uluguruensis. The females reach a maximum length of 64 mm, the males remain clearly smaller with maximal length at 51 mm. The short tail is not prehensile and amounts to an average of 20% of the body length. The head has a flat casque, which is recognizable by the coarse raised lateral edges. A small flexible and often bent down rostral process with a length of 0.08 inch (2 mm) rises forward off the snout. Two other canthal processes (supraocular appendage) are present above each eye. A crenulated dorsal crest is formed by clusters of small conical tubercles. The gular or ventral crests are not present. Each axilla have a deep dermal pit but there are no traces of a pit in the goin. Their basic color is between dark and light brown with its characteristic two dark, diagonal stripes on the flanks. Some specimen have pale green or pale yellow flanks, whereas the inside of the thigh is often auburn. Males and females frequently display light blue or turquoise eye turrets and their eyeball are orange.

These species is closely related to R. (Rd.) uluguruensis. It differs from R. (Rd.) uluguruensis principally in the number of scales of the interorbital row and the hemipenis morphology. The soles of R. (Rd.) moyeri are with smooth "cobblestoned" palms and the body scalation is of fine subhomogeneous stellate granules (MENEGON et al 2002).

Habitat
This species is found only in a small area inside a dense montane forest with the canopy ranging from 82 to 131 feet (25 to 40 m). During the night the chameleons are observed in the dense undergrowth on ferns, stems and branches at a height from 12 to 118 inches (30 to 300 cm) from the leaf litter (MENEGON et al 2002). Possible species within the montane area of the Udzungwas are Ch. (T.) laterispinis, Ch. (T.) tempeli, Ch. (T.) werneri.

Captive Care
Semi-screened enclosures are highly recommended with a cage size of 16 x 16 x 16 inches (40 x 40 x 40 cm) for a single animal. The interspecific aggression is well developed. Temperatures should be around 68°F (20°C) during the day with a maximum of 73°F (23°C). he night drop lows are under 61°F (16°C). These high fluctuations between daytime and nighttime temperatures are essential for these animals. For a humidity of 75 to 95% during the day and 90 to 100 % at night, the enclosure should be regularly misting three times a day and once more after lights off. Only a single white fluorescent tube is required over the length of the enclosure. Basking and UVB bulbs don’t seem to be necessary. Plants with good, dense leaf cover are important in maintaining the required high humidity level. A substrate of a forest type should be made with a mix of soil and sand (2:1) with a layer of dead leaves. Thin branches with dead leaves and some overgrown with tresses should be placed for climbing; this species will rest on them, depending on their camouflage for defense. Adult animals are fed every other day with appropriately sized crickets, grey roaches, flies, firebrats and waxworms. All food items should be coated every second feeding with multi mineral supplement (Miner-all) contains Vitamin D3 for proper calcium utilization. They drink the water droplets of dense plants or and absorb the surface-humidity.

Reproduction
A gravid wild caught female laid a clutch of five white eggs with a size of 0.35 x 0.16 inches (0.9 x 0.4 cm). Before laying, she carried out some test excavations at different places and decided for a proper moisture spot under the leaf litter. The egg-laying substrate should be around 3 inches (75 mm) deep. Mating was observed once by MENEGON et al (2002). The eggs from R. (Rd.) moyeri will need to be incubated in Vermiculite at cool temperatures from 64 to 68°F (18 to 20°C) with a maximum of 73°F (23°C), and a minimum of 59°F (15°C). The only clutch of these five eggs were hatched after 99 days and survived. Due to the fact that all hatchlings were males and the loss of the breeding female, an abrupt stop of reproduction in this species occurred. The clutches of ALEXANDRA BUSCH and DIRK GRAEBER (2005) incubated successfully under similar conditions accept a temperature minimum of 55°F (13°C) in winter. They hatched their clutches from four to eight eggs around 90 days. MENEGON et al (2002) documented a clutch size of three to four eggs.

Care of Hatchlings
Newborn R. (Rd.) moyeri are 0.6 inch (15 mm) in length, from snout to the tail tip. They should be housed at first in small enclosures and can be care for under the same conditions as the adults. Ambient temperature from a high of 68°F (20°C) should not be crossed in the first weeks. From an early age neonates already display defense behaviors, showing their wide open mouth to display their orange mucous membrane. It is therefore best to housed them individually. First housing should be a small enclosure of 4 x 4 x 6 inches (10 x 10 x 15 cm) with tiny twigs or blades of grass. Soil layer is required, along with a variety of small plants and ground cover such as dead leaves to hold humidity up and provide hiding places. The young are as weakly active as the adults; they often rest for hours up in the ferns or the branches with dead leaf. Regularly misting of the enclosure with a minimum of three times a day may be necessary. Dense or drooping plants like small ferns and herbs provide hiding sites for the young lizards. Hatchlings and juveniles are fed every day with as many pinhead crickets and flightless fruit flies they will eat. Additional they drink droplets off of plants, especially of ferns. At the age of six months the young can move in bigger enclosures (8 x 8 x 8 inches, 20 x 20 x 20 cm), and after 12 months transferred to the enclosure size of the adults.


Common and Scientific Names
Both the herpetological study and the care of chameleons would be impossible without a reliable system of naming. It is essential that one species has only one name used by scientists and interested people throughout the world. This allows people to be confident that they are talking about the same species. It allows a body of information to be built up about a chameleon that can be made available in books and databases. Common names are not reliable, because there is no guideline for using. They are not consistent and are very loosely applied. For example the Tanzanian stump-tailed chameleon Rieppeleon brevicaudatus has a lot of common names: Bearded Chameleon or Bearded Pygmy-Chameleon, Leaf chameleon or Leaf Pygmy-Chameleon, Usambara Leaf Chameleon, Short-tailed Pygmy-Chameleon and so on. These names are not used only for Rieppeleon brevicaudatus some of them used for R. (R.) temporalis too.
Table 2
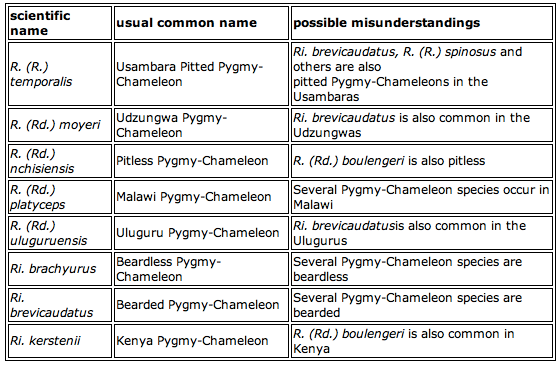
So-called “People's Names” are common names built with a part of the scientific name - the Boulenger's Chameleon is Rhampholeon (Rd.) boulengeri. It seems at first sight a reliable name for chameleon species. But the Peyrierras's Chameleon could be both Brookesia peyrierrasi or Calumma peyrierrasi. Misunderstandings are bound to happen! (table 2)
The naming system used by scientists and herpetological amateurs likewise is the binomial system of scientific names. The names are not accepted until they appear in print with a full description of the species. Scientific names follow a strict set of rules adopted by the International Commission on Zoological Nomenclature, and published in the International Code of Zoological Nomenclature. The intent of the code is to encourage stability, accuracy, and universality of an organism's scientific name.
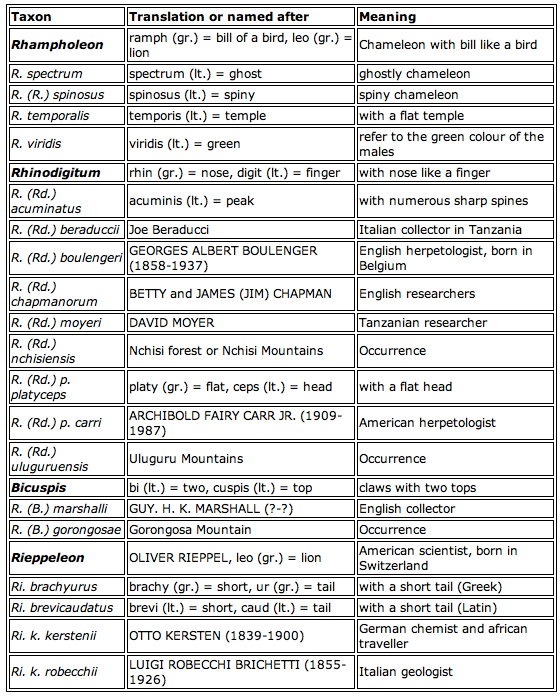
Etymology of the scientific names
Special Thanks
I would like to thank the following stump-tailed chameleon keepers and experts without whom I could not realize this article. First I would like to thank ULRIKE WALBRÖL, ROLF MÜLLER, ALEXANDRA BUSCH, DIRK GRAEBER, WOLFGANG SCHMIDT from Germany and CARL CATTAU from USA. Thank you for the exchange of your experience. I'd also like to thank STEPHAN KALLAS, Germany and TREVOR DELL, USA for their adjuvant information from the field. Especially, I would like to thank my brother JENS HILDENHAGEN for his support to make this English article possible.
Sources Cited:
BUSCH, A & D. GRAEBER. (2005): Zur Haltung und Zucht von Rhampholeon moyeri (MENEGON, SALVIDIO & TILBURY, 2002). - CHAMAELEO, Mitteilungsblatt der AG Chamäleons in der DGHT, 30: 28-30. (Care and breeding of Rhampholeon moyeri. - CHAMAELEO, bulletin of the Working Group Chameleons within the DGHT, German Society for Herpetology and Herpetoculture).
CRIBB, P. J. & G. P. LEEDAL (1982): The Mountain Flowers of Southern Tanzania: A Field Guide to the Common Flowers. - A. A. Balkema, Rotterdam: 244 pp.
DOWSETT-LEMAIRE, F. (1989): The flora and phytogeography of the evergreen forests of Malawi I: Afromontane and mid-altitude forests. - Bull. Jard. Bot. Nat. Belg., 59: 3-131.
EMMETT, D. A. (2004): Altitudinal distrubution of the short-tailed pygmy chameleon (Rhampholeon brevicaudatus) and the Usambara pitted pygmy chameleon (R. temporalis) in Tanzania. - Afr. Herp. News, 37: 12-13.
IVERSEN, S. T. (1991): The Usambara Mountains, NE Tanzania: History, vegetation and conservation. - Publication at the Uppsala universitet: 146 pp.
KÖHLER, G. (2005): Incubation of Reptile Eggs. - Krieger Publishing Company, Malabar, Florida: 214 pp.
LOVERIDGE, A. 1953. Zoological results of a fifth expedition to East Africa. III. Reptiles from Nyasaland and Tete. Bull. Mus. Comp. Zool., Havard 110(3): 142-322.
Mariaux, J. & C. Tilbury (2006): The Pygmy Chameleons of the Eastern Arc Range (Tanzania): Evolutionary relationship and the description of three new species of Rhampholeon (Sauria: Chamaeleonidae). - Herp. J. 16: 315-331.
MATTHEE, C., C. R. TILBURY & T. TOWNSEND (2004): A phylogenetic review of the African-leaf chameleons: genus Rhampholeon (Chamaeleonidae): the role of vicariance and climate change in speciation. - Proc. Roy. Soc., London B 271: 1967-1975.
MCKONE, D. J. & V. P. WALZEM (1994): A Brief Survey of Catchment Forest Reserves Mbeya Region, Tanzania. Government of Tansania/EEC Agroforesty, Soil and Water Conservation Projekt, Regional Natural Resources Office Mbeya, Tanzania. - http://www.mckone.org/mbeyacfr.html
MENEGON, M., S. SALVIDIO & C. R. TILBURY (2002): A new dwarf Chameleon from the Udzungwa Mts. Of Tanzania, East Afrika, (Squamata: Rhampholeon GÜNTHER, 1874). - Journal of Herpetology 36(1): 51-57.
NECAS, P. & W. SCHMIDT 2004. Stump-tailed Chameleons - Miniature Dragons of the Rainforests. Chimaira, Frankfurt/Main 2004: 256 pp.
SCHMIDT, W. (2004): Über das weniger bekannte Usambara-Stummelschwanzchamäleon Rhampholeon temporalis (MATSCHIE, 1892). - CHAMAELEO, Mitteilungsblatt der AG Chamäleons in der DGHT, 29: 33-35. (About the poorly known Usambara Stump-tailed chameleon Rhampholeon temporalis . - CHAMAELEO, bulletin of the Working Group Chameleons within the DGHT, German Society for Herpetology and Herpetoculture).
SHANGALI, C. F., C. K. MABULA & C. MMARI (1998): Biodiversity and human activities in the Udzungwa Mountain forests, Tanzania. I. Ethnobotanical survey in the Udzungwa Scarp Forest Reserve. â€- J. East Afr. Nat. Hist. 87: 291-318.
SPAWLS, S., K. HOWELL, R. DREWES & J. ASHE (2002): A Field Guide to the Reptiles of East Africa. - Academic Press, New York: 543 pp.
TILBURY, C. R. & J. MARIAUX (2004): A re-evaluation of the generic status of Bradypodion spinosum (MATSCHIE 1892) and some consideration in the genus Rhampholeon GÜNTHER, 1874. - Rev Suisse Zool. 111: 103 - 110.

Thomas Hildenhagen

Thomas Hildenhagen is a 41 year old chameleon breeder from Germany. He has been keeping and breeding various reptile and amphibian speices for over 25 years and chameleons for more then 15 years. His current focus is on the stump-tailed chameleons and other true chameleons from Tanzania. He is a member of the Chameleon Working Group board (AG Chamäleons) within the DGHT (German Society for Herpetology and Herpetoculture, presented by the newsletter CHAMAELEO) and the community of interests of chameleons (IG-Chamaeleons). He can be emailed at thomas.hildenhagen@chamaeleons.org.









Join Our Facebook Page for Updates on New Issues:
© 2002-2014 Chameleonnews.com All rights reserved.
Reproduction in whole or part expressly forbidden without permission from the publisher. For permission, please contact the editor at editor@chameleonnews.com
